2023 – the “year of automation” in sample transportation with the DEBATIN innovation, DEBAMED® Speci-Sorb
Biological and medical sample packaging, the ADR and the safe solution
Safe “laboratory sample transportation” – whether to or from the lab – follows the strict regulations of the ADR – the European Agreement concerning the International Carriage of Dangerous Goods by Road. And when does the ADR apply to transportation? Whenever dangerous goods that are transported by road are involved.
Where dangers have to be averted, the regulations often do not leave much leeway for optimising processes in terms of the packaging for medical samples, which is part of sample transportation.
With DEBATIN’s innovative new product, DEBAMED® Speci-Sorb, you can make optimal use of this leeway in the logistics process for your sample transportation. An essential requirement in the logistics process for transporting biological samples or exempt medical specimens – from the doctor’s surgery or the hospital to the laboratory – whilst complying with the ADR transport regulations is adding an absorbent material to the secondary packaging for safe transportation or shipping. The same also applies to transportation by post, incidentally. And: DEBAMED® Speci-Sorb therefore fits in with other changes in the laboratory world – for example, how the order entry process is managed. Ultimately, all signs point to greater procedural efficiency – including during sample transportation.
You are currently viewing a placeholder content from Default. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
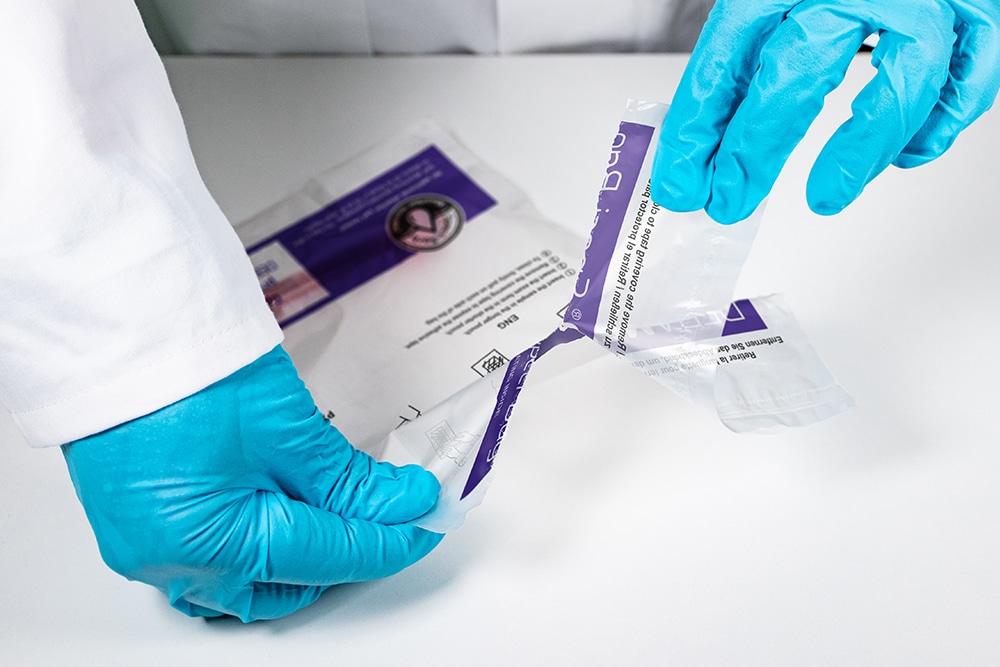
More InformationHow things used to look without DEBAMED® Speci-Sorb
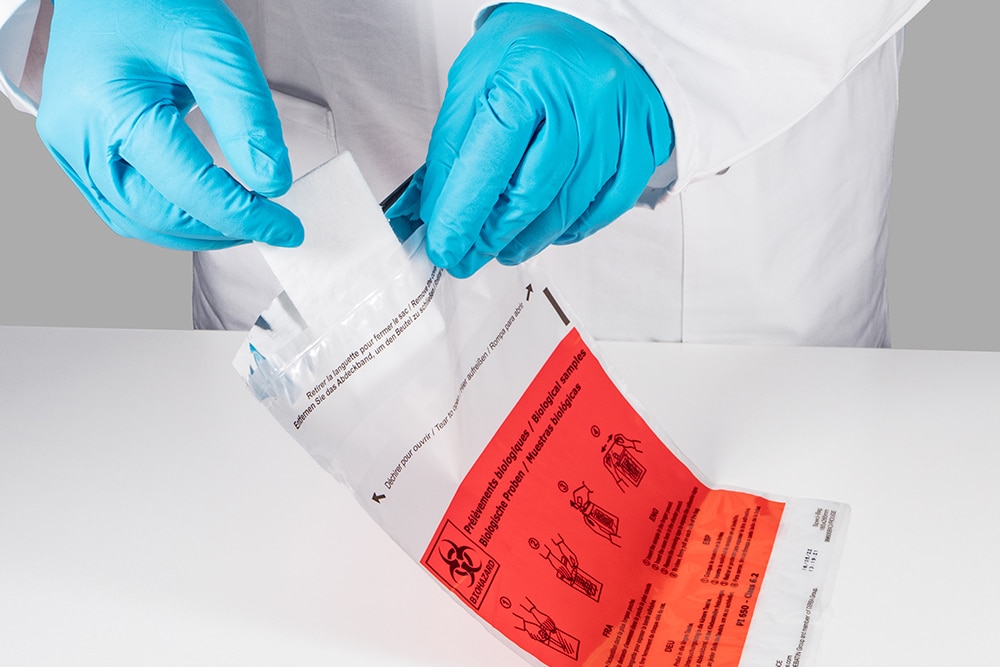
How things look now with DEBAMED® Speci-Sorb
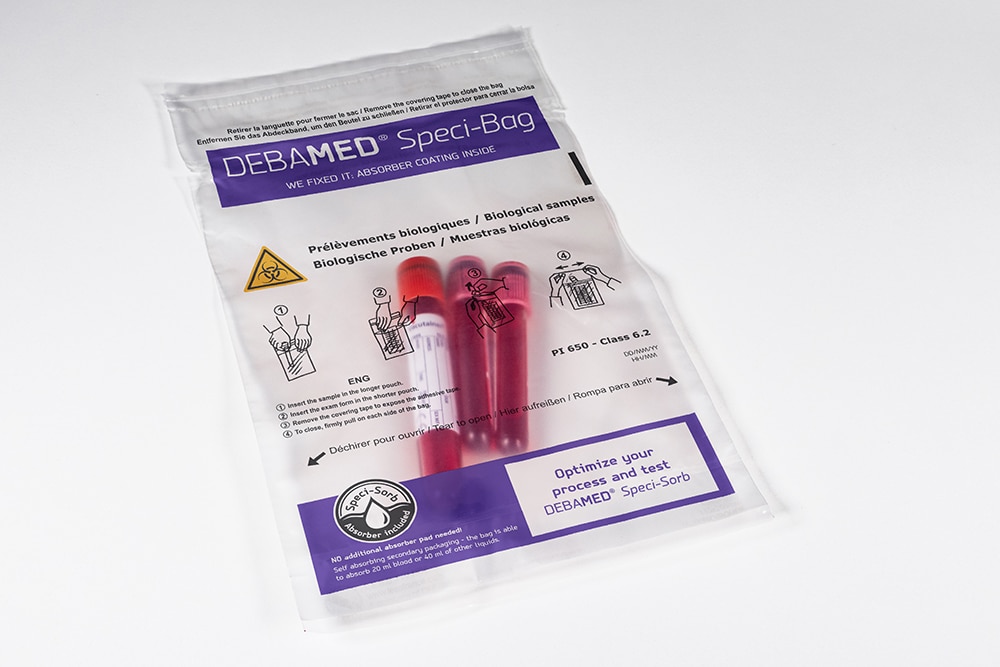
Where does DEBATIN make a difference to packaging for medical samples or when sending them to the laboratory (sample transportation)?
From 2 to 1 when packing for sample transportation!
The DEBATIN innovation: The absorbent material is applied directly to the secondary packaging in the form of a powder. As a solution provider for our customers and partners, we’ve drawn on our decades of expertise in the field of transporting biological and medical samples (indeed, sample transportation in general) to come up with this innovative coating – the patent is pending.
The benefits that we pack in beforehand for your sample transportation:
- You put NOTHING in ⇒ No manual packing process in your laboratory
- WE don’t put anything in either ⇒ No manual packing process that we would have to charge you for
- Everything that is important for ADR-compliant sample transportation is in there, which means the following benefits are now included:
- More safety and peace of mind, because nothing can be forgotten when it comes to biological and medical sample transportation
- More hygiene to protect your laboratory staff, because nothing can leak out any more
- More economy, because one step in the sample transportation process is no longer necessary
- More sustainable sample transportation, because the majority of the packaging goes into recycling
- More sustainable transportation of samples, because the entire packaging can go back into the recycling loop
- Mehr ore sense, because we understand your needs: this way, your experts can take care of the really important things in your laboratory and relating to preanalytics, medical samples and sample transportation
Why have we developed DEBAMED® Speci-Sorb for sample transportation?
We are well versed in everything that has to be done during sample transportation to and from the laboratory – because it is hugely important to genuinely observe packaging instruction P650. That is why we have also packed everything worth knowing about packaging instruction P650 and laboratory sample transportation into one of the most widely read white papers on this subject, which you can download here: request Whitepaper. For safe sample transportation and the transportation of specimens in day-to-day clinical practice!
Our experiences from the laboratory world tell us that, despite the regulations, the required absorbent liner is not always included when transporting infectious material to a laboratory (transportation of specimens)… Even though it MUST be included if you are to package your biological samples correctly for transport. That is important above all because (at least in Germany) the law on infection protection stipulates a reverse onus provision if there is an incident during sample transportation. “Laboratory sample transportation” is therefore a task to which due attention should be paid.
What duty do you have when transporting samples subject to labelling under the ADR?
The ADR makes an absorbent liner mandatory in the secondary packaging. If you leave the absorbent liner out (by forgetting it or on purpose), if the worst comes to the worst you will be obliged to prove that sending your medical samples “without” was just as safe as with an absorbent liner and was therefore done according to regulations. It’s an unnecessary risk – and one that we simply eliminate for you with DEBAMED® Speci-Sorb! The DEBATIN slogan, “Safe. Perfect. Packed.” also applies to the transportation of specimens.
Why?
We have a machine to apply the coating to the inside of the DEBAMED® Speci-Sorb. You no longer have to think about absorbent liners and, what’s more, nor do you have to procure and insert absorbent liners separately. This is a solution that has been registered at the patent office. It is innovative, reliable, straightforward, clean, simple and guarantees compliant sample transportation! Not only does that benefit your employees, but it also increases the safety of the people whose job it is to transport your samples.
Safe. Perfect. LEGAL. Packed. “Laboratory sample transportation”, fully compliant with regulations!
Sample transportation done the smart way!
In times of an acute shortage of personnel, we want to help you stop holding up your experts in the laboratory with jobs that do not belong to their key skills. However, since safety and sustainability also take priority in the laboratory and have an impact on your core competences, we have developed a smart solution for sample transportation in the form of DEBAMED® Speci-Sorb. This eliminates unnecessary work steps and at the same time counts as a credit on your laboratory’s sustainability account. This works perfectly well when transporting specimens.
Why is DEBAMED® Speci-Sorb more sustainable than the solo absorbent liner when transporting a specimen?
Our DDEBAMED® products are sustainable, because they can be recycled. Before any ADR-compliant secondary packaging can be recycled, however, the transport bag and absorbent liner have to be separated once the sample has been transported. That is another work step that is saved with DEBAMED® Speci-Sorb. The carbon-neutral DEBATIN products thus reduce your carbon footprint even faster and in a more process-oriented manner. In summary: our products represent state of the art packaging, transport packaging and sample transportation / laboratory sample transportation – made for the future of sample transportation from the surgery to the laboratory!
The coating in DEBAMED® Speci-Sorb transforms your secondary packaging into a technologically complex solution, which also affects how you go about recycling it after use. In practice, the entire packaging – including the part that’s coated – can now be channelled back into the recycling loop. The recyclability of DEBAMED® Speci-Sorb packaging has been verified and certified by the interseroh Recycling Alliance. It was tested by IVV (Fraunhofer Institute for Process Engineering and Packaging) and shown to meet the assessment criteria and standards published by German environmental institute bifa.
The low density polyethylene (LDPE) we use in our manufacturing is easy to separate prior to recycling via a simple sink/float procedure. LDPE is less dense than water, while the absorbent layer is denser than water. As a result, the Speci-Sorb coating is reliably flushed away during the flotation separation procedure that always precedes the actual recycling process.
The packaging scored 2 out of 2 points for being easy to assign in the collection system, 6 out of 6 points for being easy to sort and 8 out of 12 points for how suitable it is for recycling.
How does it make your life easier? Tear open the bag – and that’s it! The fiddly job of disposing of the absorbent liner is a thing of the past! The laboratory sample transportation process thus becomes part of your sustainability strategy.
FAQ about sample transportation, laboratory, medical samples, medical packaging, secondary packaging, laboratory sample transportation, etc.
When does the ADR apply to transportation?
The ADR applies within the borders of the European Union whenever dangerous goods are being transported. In Germany, the Ordinance on the Transport of Dangerous Goods by Road, Rail and Inland Waterways (GGVSEB) – formerly the Ordinance on the Transport of Dangerous Goods by Road (GGVS) – implements the ADR regulations at the national level. The GGVSEB can be viewed online.
The ADR is a relatively complex set of regulations and relates to the following topics:
- Classification
- Packaging
- Labelling
- Documentation of dangerous goods
And be aware: anyone breaching the regulations must expect a fine.
Under some circumstances, this also applies when transporting specimens to a laboratory.
| Offence | Bußgeld |
|---|---|
| Orange plate not attached or not visibly attached | 300 € |
| OOrange plate not correctly attached / not correctly visibly attached | 100 € |
| Leak tightness not checked | 250 € |
| Instructions in writing not kept in vehicle / not handed over (on time) | 150 € |
| Transport documents not kept in vehicle / not handed over (on time) | 150 € |
| Certificate of participation on basic course or advanced course not kept in vehicle / not handed over (on time) | 500 € |
| RNo smoking rule not observed | 250 € |
| Not cleaned after unloading | 250 € |
| Loading or unloading where not permitted | 100 € |
Categorisation of infectious substances:
Infectious substances are divided into categories for transportation, based on their hazard potential, which is essentially defined by the pathogenicity and infectivity of the particular pathogens. They are subdivided further based on the type of infectious material. Medical test materials can be divided into:
- Patient samples
- Exempt medical specimens
- Microbial cultures
Other significant groups of infectious materials from a health perspective are medical waste (hospital, laboratory and surgery waste) and also biological products (vaccines, antisera, diagnostic products) where applicable.
Proper classification of infectious substances is fundamentally important to how they are further treated as dangerous goods in compliance with regulations.
The packaging regulations – and all other regulations to be complied with during transportation – are derived from the UN number determined as a result of the classification.
What is UN 2814?
The United Nations have defined two sub-categories in the ADR: Category A and Category B. UN 2814 (affecting humans) and UN 2900 (affecting animals only) are further sub-categories of A.
UN 2814 comes into force whenever sample transportation involves an infectious substance which is carried in a form that, when exposure to it occurs, is capable of causing permanent disability, life threatening or fatal disease in otherwise healthy humans or animals. Packaging regulation P620 applies in this case.
What is UN 3373?
The subdivision of the ADR into Category A and Category B gives rise to further sub-categories (see the question “What is UN 2814?”).
Category B is known as UN 3337 and is described as: “any infectious substance that does not meet the criteria for inclusion in category A”.
What is meant by primary and secondary packaging?
When transporting medical samples, you may need outer packaging as well as primary and secondary packaging. All three types of packaging are required when transporting biological samples and for sample transportation to a laboratory.
- The primary packaging is a watertight primary container (e.g. Monovette)
- The secondary packaging must be watertight and leakproof
- The outer packaging must be adequately strong (at least one surface with dimensions of 100 × 100 mm)
The secondary packaging must be inserted in the outer packaging with suitable padding material. For liquid substances, sufficiently absorbent material must also be inserted between the primary and secondary packaging.
The secondary packaging or the outer packaging must be rigid.
Primary or secondary packaging must be able to withstand an internal pressure of 95 kPa without leakage or loss of content.
Which regulations apply to sample transportation?
- ADR
- IATA
- RID
- IMDG
Which authorities are important when it comes to sample transportation?
- Robert Koch-Institut (RKI)
- Friedrich-Löffler-Institut (FLI)
- Federal Institute for Occupational Safety and Health (BAUA)
- Employers’ liability insurance associations
What packaging regulations apply to sample transportation?
- P 620
- P 650
- Rules for exempted human/animal specimens in section 6.2 of ADR 2023
What quality labelling is there for packaging – especially for laboratory sample transportation?
- Bundesanstalt für Materialforschung und -prüfung (BAM)
- Belgian Packaging Institut (IBE-BVI)
- BVT (Bureau de Vérifications Techniques)